How Many Electrons Are in the Outermost Shell of Phosphorus
It is also a transition element but it doesnt show variable valences. C The atoms lose their electrons more easily.
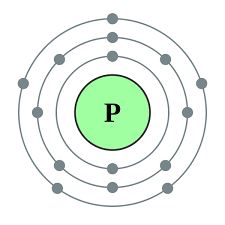
Phosphorus Valence Electrons Phosphorus Valency P With Dot Diagram
The ground-state electron configuration of an atom is 1s22s22p63s23p4.
. Fluorine with nine electrons carries two in its first shell and seven in the second. Lewis structure of boron trifluoride. The 7th IE is the largest number because at that point you are trying to remove the 2s2 electrons from a full valence shell.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. For atoms the notation consists of a sequence of atomic subshell labels eg. If an element X is placed in group 14 it has 4 electrons in its outermost orbit.
Hence the electron configuration for Ca2 is 1s22s22p63s23p6. How many electrons must be gained or lost to achieve a noble gas. Its electronic configuration is 285.
Its electronic configuration is Ar. Each pair of dots represents a pair of electrons. These are called the valence electrons and they are the electrons that can chemically bond with.
Thus its valency is 3. A bond can be drawn as a line between two. B The number of valence electrons increases.
Phosphorus P is also in Group VA which means it also has five electrons in its outer orbital. In this compound the boron atom only has six valence shell electrons but the octet rule is satisfied by the fluorine atoms. So the mutual shielding of 3d and 4s electrons is weak and the effective nuclear charge acting.
For phosphorus the sequence 1s 2s 2p 3s 3p with the number of electrons assigned to each subshell placed as a superscript. Whereas these two electrons lost by Mg are gained by oxygen to complete its stable octet. The magnesium element has 2 electrons in outermost orbit.
However because the atomic number for phosphorus is fifteen the electron configuration is 2-8-5. What is the valence-shell configuration of the atom in the same group but in Period 5. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
Periodic Table of Elements 1. Meanwhile the column or group signifies the number of electrons in the atoms outermost shell. Formula of its Chloride is ECl 4.
Phosphorus Atomic number of phosphorus is 15. Periodic Table of Elements 3. Neon with ten electrons carries two in the first and eight in the second.
X would most likely be in the same group of the Periodic Table as. Total valence electron in Phosphorous 5. The 3d orbital forms part of the n3 shell whose average position is closer to the nucleus than the 4s orbital and the n4 shell but electrons in s orbitals experience greater penetration into the nucleus than electrons in d orbitals.
The 4s and 3d electrons have similar shielding ability. When placed between two atoms the electrons are in a bond. Give reasons for your answer.
Valence electrons can also be determined as the electrons present in the shell with highest principal quantum number n. Physicists and chemists use a standard notation to indicate the electron configurations of atoms and molecules. A X has 12 protons and 12 electrons b Y has 12 protons and 10.
Total valence electron in Fluorine 7. Here element can form compound by sharing electrons hence it will be a chemical bonding. For example The electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2.
Loss of how many electrons The largest ionization jump will occur following the loss of the 6th e-. The magnesium has two electrons in its outermost orbit ie M shell and oxygen needs two electrons to form a stable octet. The total number of electrons is the same as the atomic number for Cl 17 its in notice that Sn is in the part of the table columns 13-18 where the p-orbitals are filling since its in the 5th column it has 5 valence electrons in the p orbitals.
The Mg loses two electrons and forms a stable octet with 12 protons and 10 electrons in the L shell. D The oxides become more acidic. Phosphorous atom belongs to Group 5A or 15A in the periodic table hence it has a 5 valence electron in its outermost shell whereas fluorine atom belongs to Group 7A or 17A hence it has a 7 valence electron in its outermost shell.
As an opposite charge is being. Hence it has 2 valence electrons. Enter the email address you signed up with and well email you a reset link.
Lone pair electrons have the maximum repulsion and bond pair electrons the minimum. Compare the radii of two species X and Y. Multiple bonds are accounted as single electron pairs and bonded electron pairs as a single pair.
How many valence electrons are in an atom of. C The atoms lose their electrons more easily. However because the atomic number for phosphorus.
Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all. The electrons in the outermost valence. If p-oritals are filling then the s-orbitals are already full add 2 more valence electrons.
Since we need to take away two electrons we first remove electrons from the outermost shell n4. All electron pairs assume positions of least repulsion. How many electrons does barium have to give up to achieve a noble gas.
Gold silver helium oxygen mercury hydrogen sodium nitrogen niobium neodymium chlorine carbon. The valence or outermost electron shell is assumed to be spherical. Because the number of electrons in the second shell increases we can begin to imagine why the chemical properties gradually change as we move from oxygen to fluorine to neon.
Element X forms a chloride with the formula XCl2 which is a solid with a high melting point. How can you tell from a list of ionization energies for an element where a kernel non valence electron has been. For example hydrogen has one electron in the s-orbital of the.
So it needs 3 electrons to fulfill its outermost shell and attain stability. Scandium Atomic number of scandium is 21.

How Many Electrons Does Outer Shell Of Phosphorus Have Brainly In

How Many Electrons Does Outer Shell Of Phosphorus Have
No comments for "How Many Electrons Are in the Outermost Shell of Phosphorus"
Post a Comment